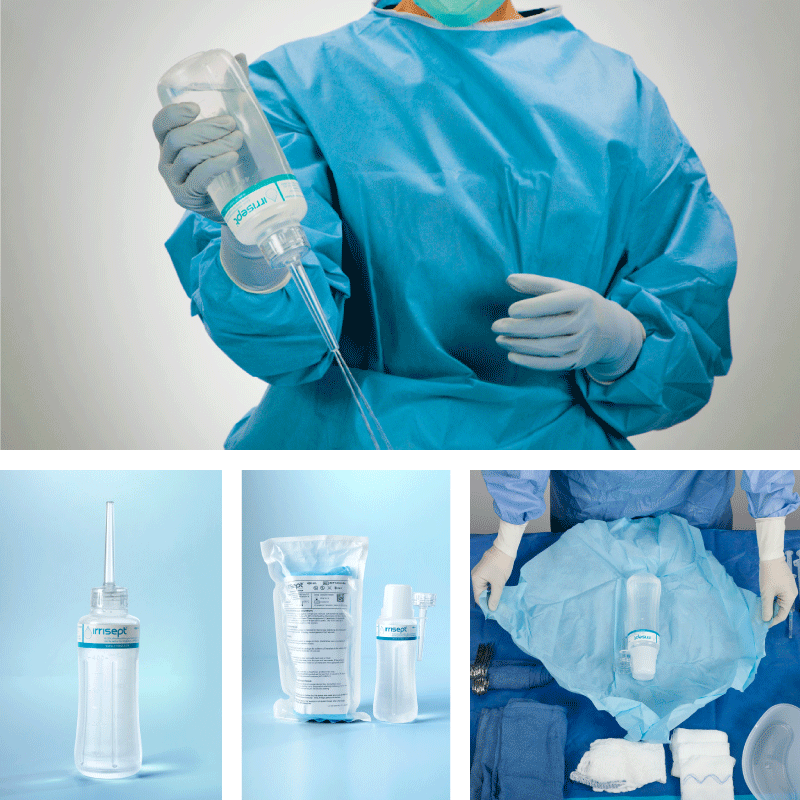
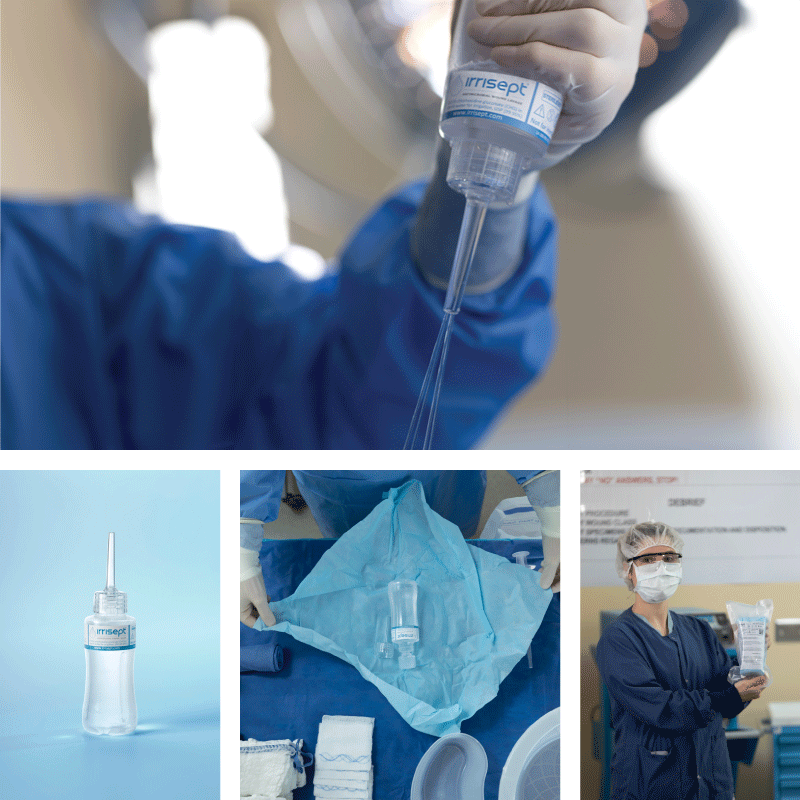
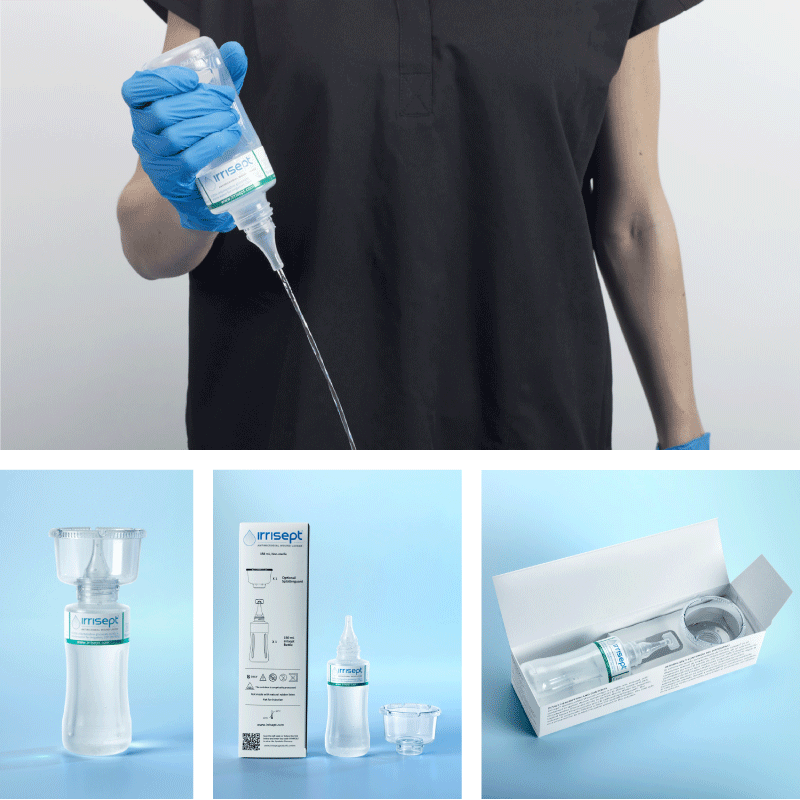
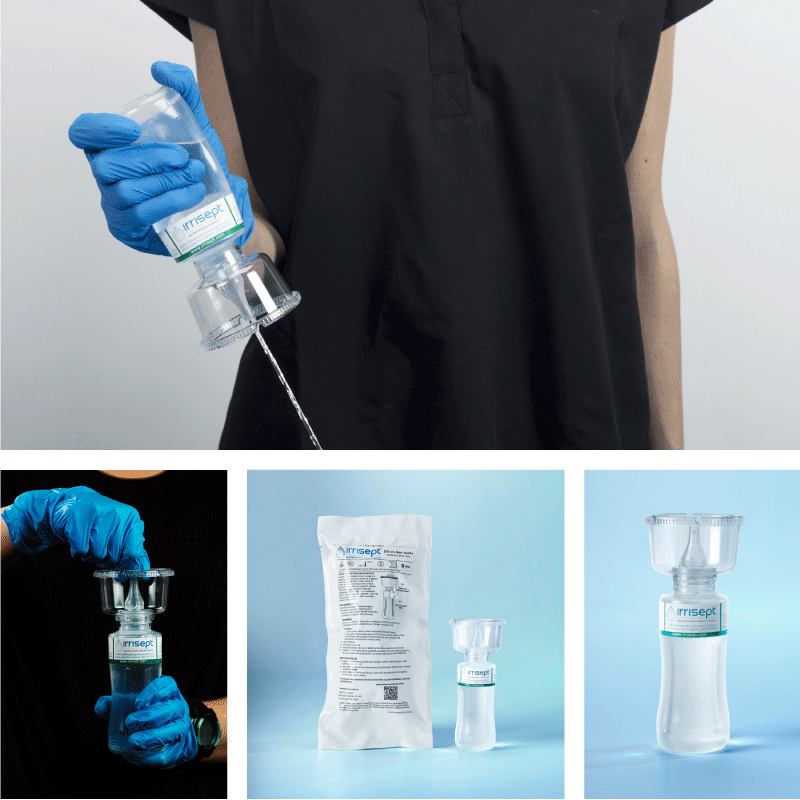
Irrisept is a self-contained jet lavage that contains chlorhexidine gluconate (CHG) as a preservative to offer broad-spectrum activity against various microorganisms in the bottled solution.1
Products
View current products and learn more about Irrisept®
A New Standard of Care for Wound Irrigation
Product Sizes/Packaging
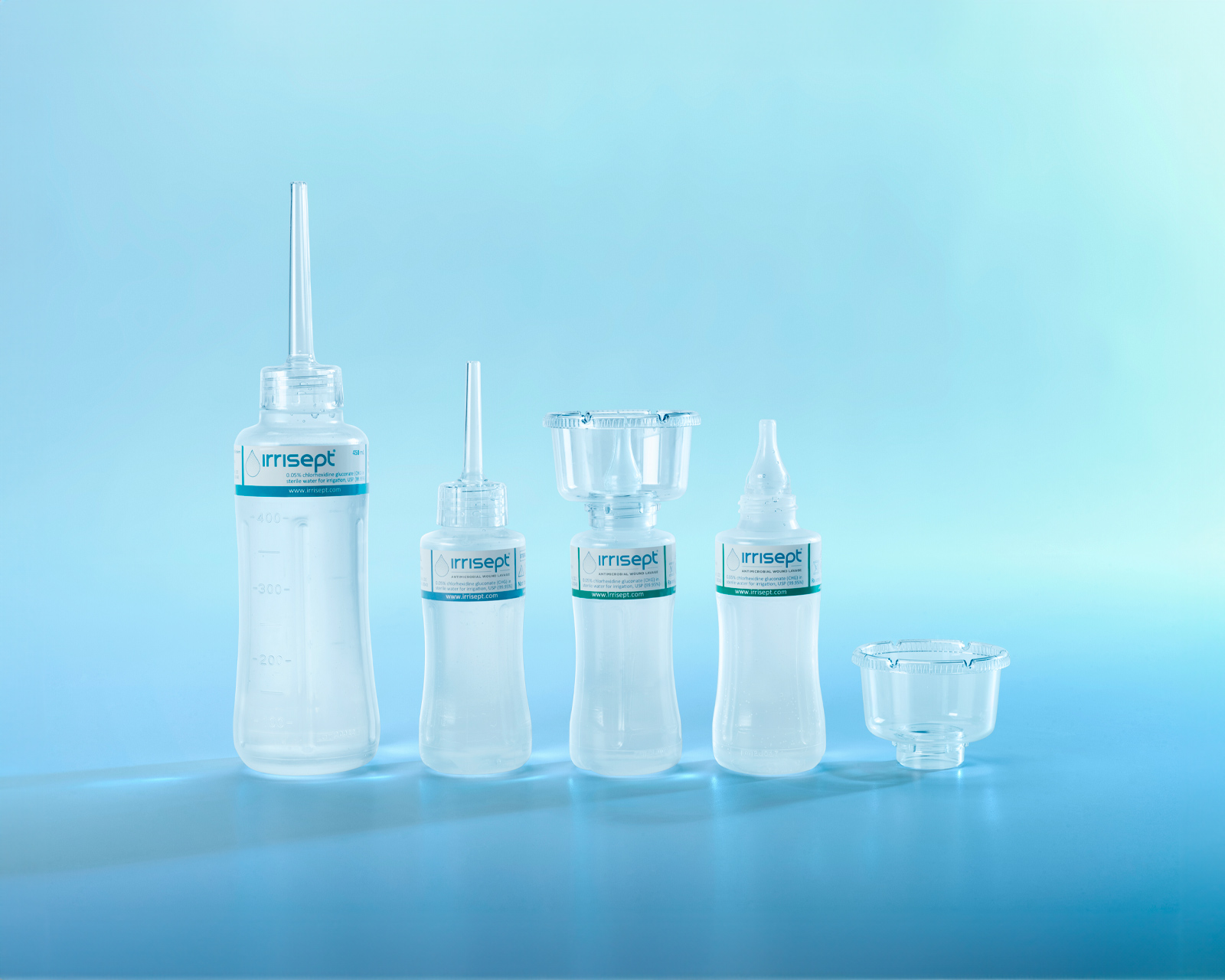
Irrisept Antimicrobial Wound Lavage is offered in a variety of sizes and configurations to meet your clinical needs.
Irrisept is intended for mechanical cleansing and removal of debris, dirt, and foreign materials, including microorganisms from wounds.
Warning: Do not use this product if the patient is allergic to chlorhexidine gluconate. Discontinue use immediately if irritation, sensitization, or allergic reaction occurs.
Irrisept Antimicrobial Wound Lavage
450 mL, Sterile Packaged
- Manufacturer #: ISEPT-450-USA
- Includes: (1) 450mL bottle of Irrisept, (1) Irriprobe applicator, and (1) Convenience cap in sterile packaging
- 12 bottles per case
Irrisept Antimicrobial Wound Lavage
150 mL, Sterile Packaged
- Manufacturer #: ISEPT-150-USA
- Includes: (1) 150mL bottle of Irrisept and (1) Irriprobe applicator in sterile packaging.
- 15 bottles per case
Irrisept Antimicrobial Wound Lavage
150 mL, Post Acute Wound Care
- Manufacturer #: ISEPT-150N-USA
- Includes: (1) 150mL bottle of Irrisept with (1) attachable Splatterguard in an easy-to-store shelf carton
- 15 bottles per case
Irrisept Antimicrobial Wound Lavage
150 mL, Tear & Go Pouch
- Manufacturer #: ISEPT-150RP-USA
- Includes: (1) 150mL bottle of Irrisept with (1) detachable Splatterguard in a Tear & Go Pouch
- 12 bottles per case
FAQ
What is Irrisept®?
Irrisept Antimicrobial Wound Lavage is a single-use, manual, self-contained irrigation device. Irrisept contains 0.05% chlorhexidine gluconate (CHG) in 99.95% Sterile Water for Irrigation, United States Pharmacopeia (USP). The solution is aseptically filled in a Blow-Fill-Seal (BFS) bottle. The CHG acts as a preservative to inhibit microbial growth in the bottled solution. Irrisept is a Class II Medical Device and an unclassified Combination Product.
What is CHG?
CHG is chlorhexidine gluconate. It is a cationic bisbiguanide salt. CHG works by destroying the bacterial cell membrane and precipitating cell contents. The attraction of the positively charged CHG molecule to negatively charged bacterial cell wall causes disruption of the cell membrane and subsequent cellular death.2 CHG acts as a preservative to help inhibit microbial growth in the bottled solution.
What are Irrisept’s indications for use in the USA?
The Irrisept Antimicrobial Wound Lavage is intended for mechanical cleansing and removal of debris, dirt, and foreign materials, including microorganisms from wounds.
How does the device create pressure for irrigation?
Irrisept’s bottle design allows you to control the delivery pressure of the solution through manual bottle compression. Grasping the bottle firmly, you can control the direction and pressure desired to effectively loosen and remove wound debris.
Where can I use Irrisept?
Irrisept can be used for all types of wounds.
Is Irrisept safe to use on wounds?
Irrisept meets biocompatibility guidelines for ≤ 24 hours contact with breached or compromised surfaces following the ISO standard 10993-1. The following testing supports biocompatibility of Irrisept for the intended use of wound cleansing and debridement3:
- Cytotoxicity
- Sensitization
- Irritation
- Acute Systemic Toxicity
- Material Mediated Pyrogens
Reports for all testing stated above are on file and available upon request through Irrimax Corporation.
What are the risks and safety information associated with use of Irrisept?
WARNINGS
- Do not use this product if the patient is allergic to chlorhexidine gluconate.
- Discontinue use immediately if irritation, sensitization, or allergic reaction occurs.
CAUTIONS
- Do not use unless solution is clear and bottle twist seal is intact.
- When using this product keep away from eyes and ear canals. If solution inadvertently contacts these areas, rinse out promptly and thoroughly with water and/or normal saline.
- Not for injection.
- Single patient use only.
- Irrisept is intended for use in adults by healthcare professionals only.
- Irrisept solution meets biocompatibility guidelines for ≤ 24 hours contact with breached or compromised surfaces (ISO 10993-1).
Why does Irrisept packaging state “Rx Only”?
Irrisept is a prescription device. FDA regulations require that prescription devices have the following statement “Caution: Federal law restricts this device to sale by or on the order of a licensed healthcare practitioner.”
Is Irrisept sterile?
ISEPT-450-USA and ISEPT-150-USA are manufactured using aseptic processing per ISO13408 Aseptic Processing of Healthcare Products. The bottle, applicator, and packaging are EO-sterilized.
ISEPT-150N-USA and ISEPT-150RP-USA are also manufactured using aseptic processing per ISO13408 Aseptic Processing of Healthcare Products.
Do I have to rinse with saline?
Labeling on all Irrisept products states: “Wait for approximately one minute, rinse with normal saline for irrigation. Discard any unused solution.”
Can Irrisept be used on more than one patient?
Labeling on all Irrisept products states: “Single patient use only” along with “single use only, do not reuse” symbol.
What are the storage condition requirements for Irrisept?
Labeling on all Irrisept products state storage temperature is between 10°–30° degrees Celsius (50°–86° Fahrenheit).
What is the shelf life of Irrisept?
Irrisept maintains a 3-year shelf life from the date of manufacture, which is indicated on the product packaging or IFU insert.
Where can I find the expiration date on the Irrisept product?
The expiration date may be located at the top of the Irrisept product pouch label or on a sticker on the pouch or box.
Can Irrisept be warmed?
If desired, the Irrisept Antimicrobial Wound Lavage device may be warmed in its packaging up to 40°C (104°F) by placing it in a temperature warming cabinet prior to use. Note: Prior to warming, store the device as per the device labeling.4
What is the pH range for Irrisept?
Each LOT of Irrisept is tested to meet a pH range of 5.0 to 7.0.
References
1. (2022). KTK Summary. Doc. 537161 V5 Evaluation of CHG as a preservative in the solution
2. Biocompatability Matrix. Data on file at Irrimax Corp. Lawrenceville, GA.
3. TR 17-017 Irrisept Temperature Excursion Test
4. McDonnell, G. & Russell, A.D. (1999). Antiseptics and Disinfectants; Activity, Action and Resistance. Clin Micro Rev, 12(1), 147-179. doi.org/10.1128/cmr.12.1.147